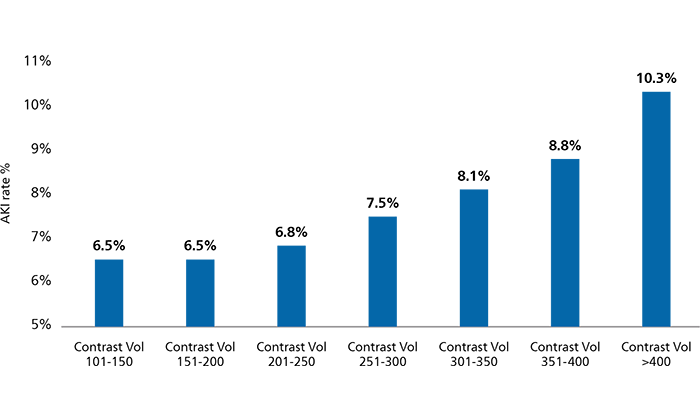pro 16, 2021 by Philips
Reading time: 4-5 minutes
Ultra low contrast PCI
Share this article
Sign up to receive news and updates from Philips
AKI is a serious complication of PCI
Most cardiac patients fall under the high-risk category for acute kidney injury (AKI). Heart failure, ST-elevation myocardial infarction (STEMI), cardiogenic shock, chronic kidney disease (CKD), > 75 years old and diabetes contribute to the higher incidence of contrast-induced nephropathy (CIN).1
AKI affects 1 in 5 hospitalized patients2
Inpatient mortality for AKI patients is 20-25%; 50% for those on dialysis3
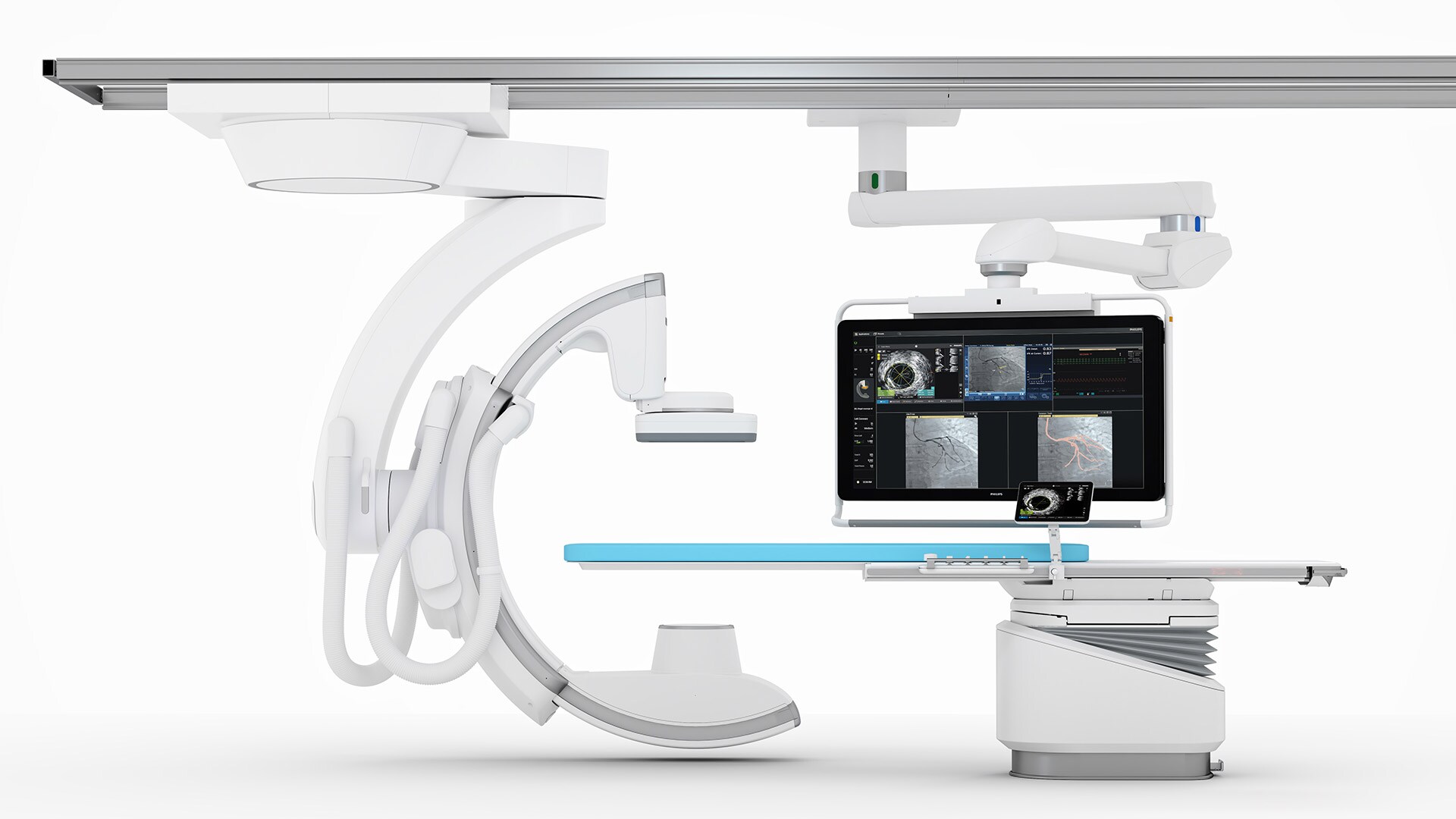
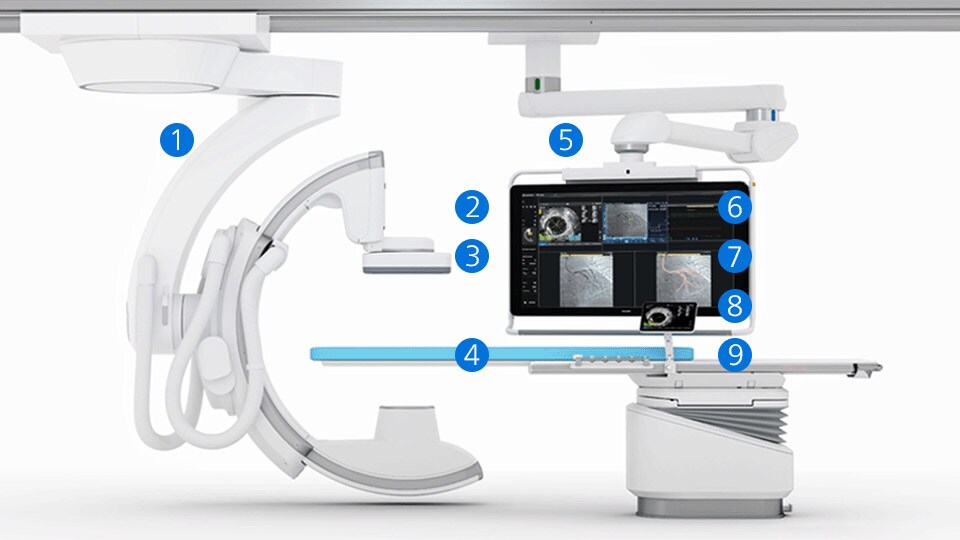
Explore the broadest suite of integrated solutions for ultra-low contrast PCI procedures
Expand each product to learn more.
-
-

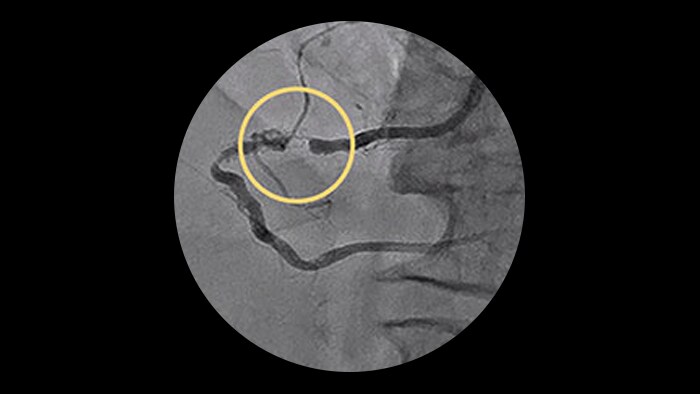
CardiacSwing
A dual-axis rotational system allows you to see comprehensive views of the coronary anatomy, including views often hidden during conventional angiography.
You are about to visit a Philips global content page
Continue -
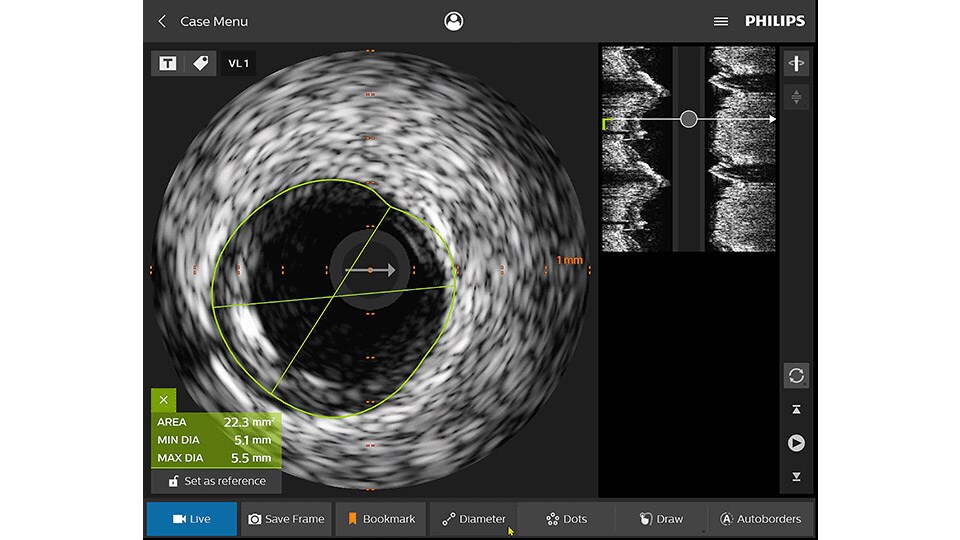
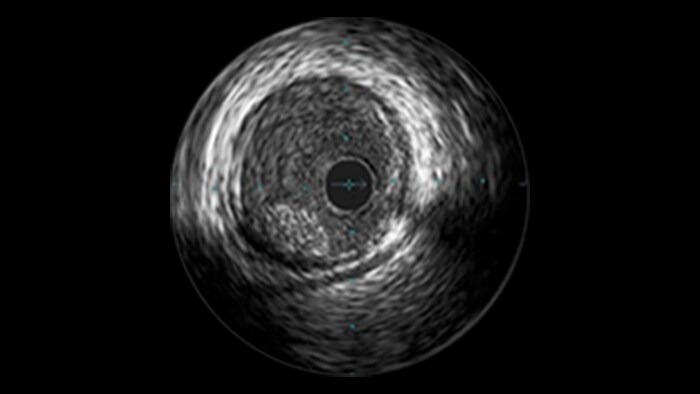
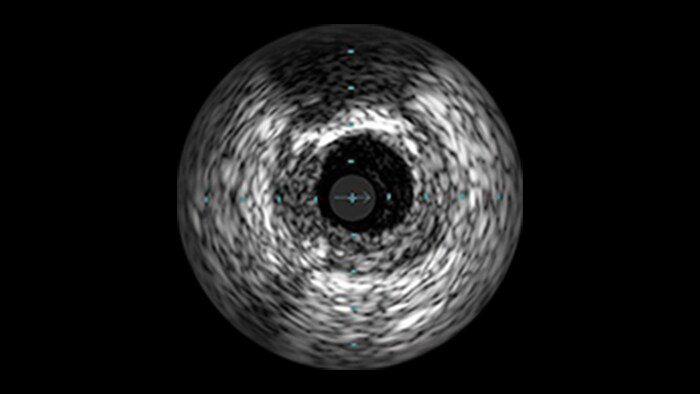
IVUS
Intravascular ultrasound (IVUS) provides images from within the vessel to accurately assess and optimize your treatment plan.
You are about to visit a Philips global content page
Continue -
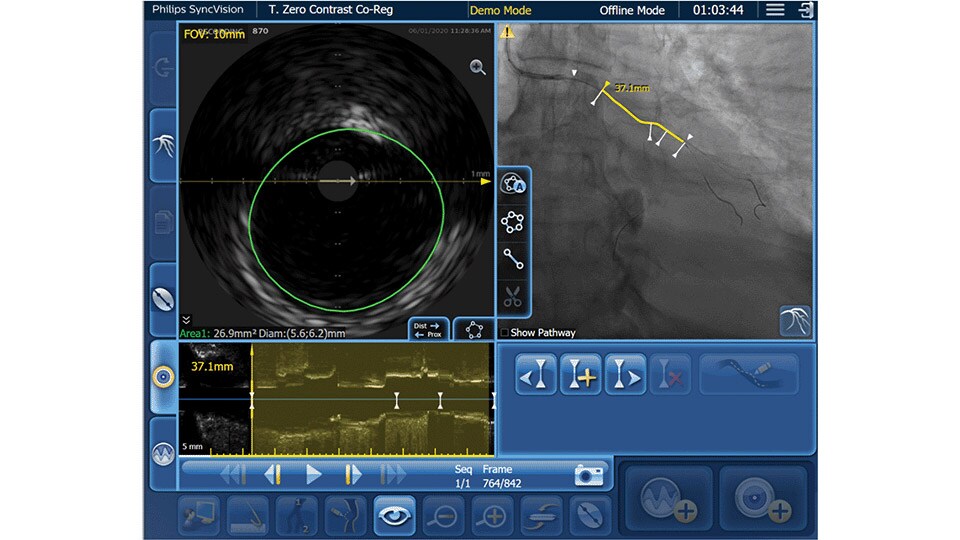
IVUS co-registration
Software helps you understand precisely where the disease begins and ends and guides your pre-and post-strategy decisions for improved outcomes.
You are about to visit a Philips global content page
Continue -
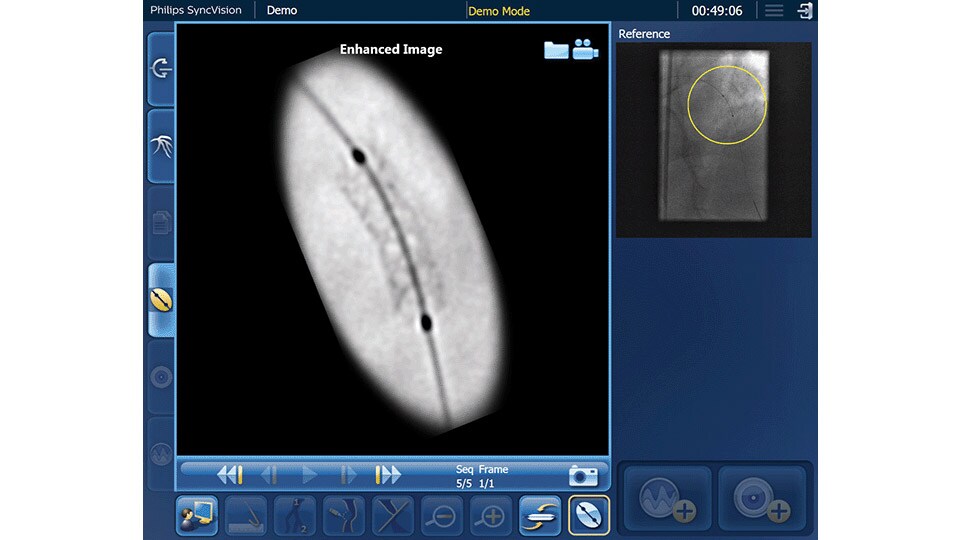
Device detection
Enhanced stent visualization quickly verifies positioning before and after deploying balloons and stents.
You are about to visit a Philips global content page
Continue -
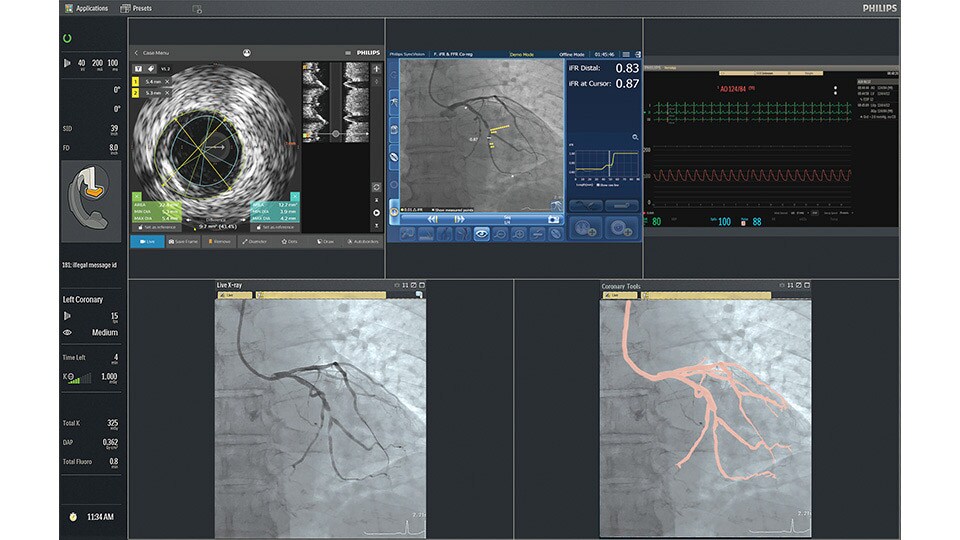
IntraSight
An interventional applications workspace that brings physiology, imaging and angiographic guidance tools together to optimize treatment plans.
You are about to visit a Philips global content page
Continue -
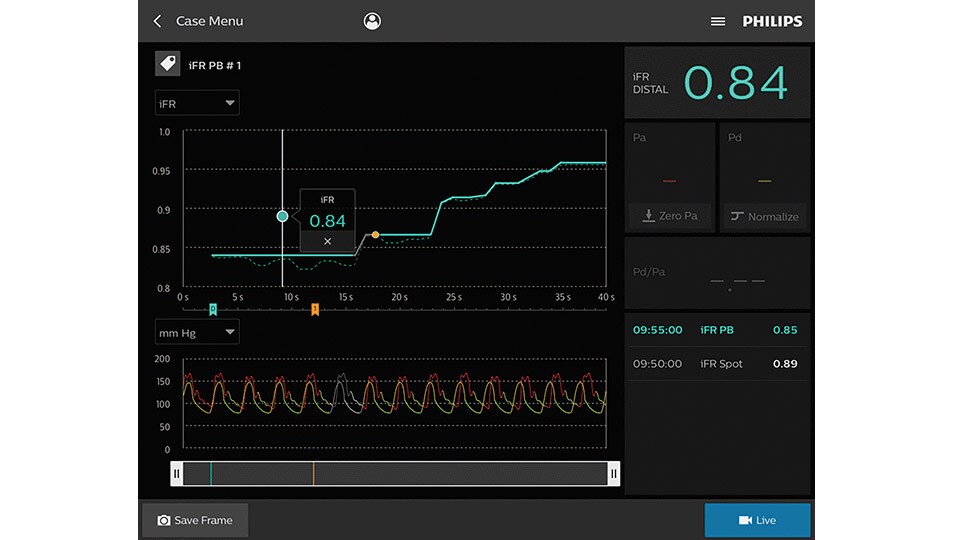
iFR
Only Philips instant-wave free radio (iFR) has co-registration for advanced physiologic guidance, allowing you to precisely determine lesion location and severity.
You are about to visit a Philips global content page
Continue -
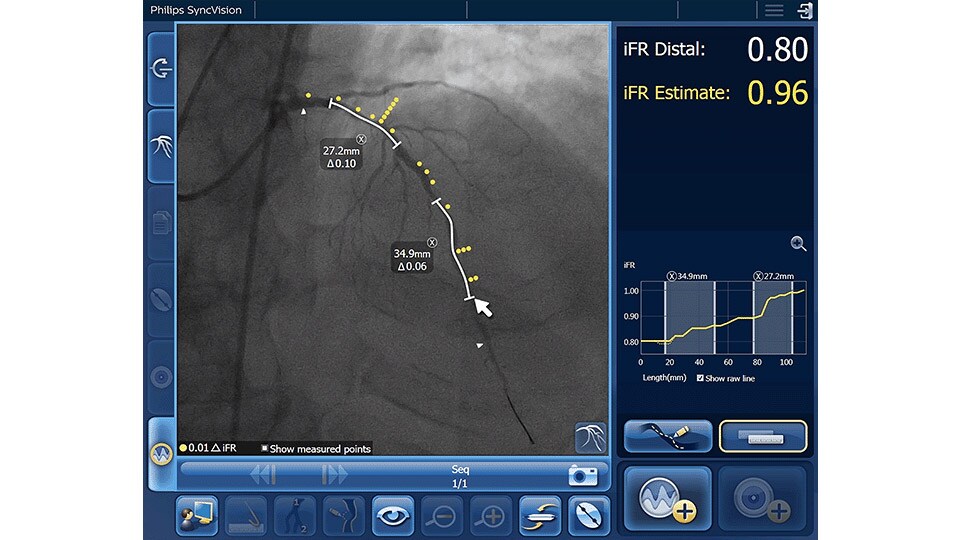
iFR Co-registration
As an alternative or adjunct to IVUS Co-registration, iFR pullback can be co-registered onto the angiogram to assess both the degree and length of vessel stenosis.
You are about to visit a Philips global content page
Continue -
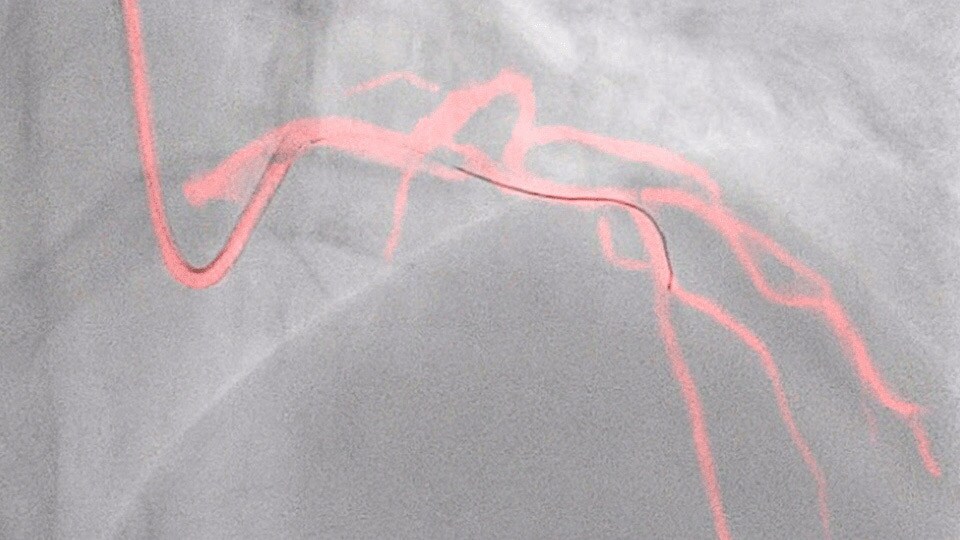
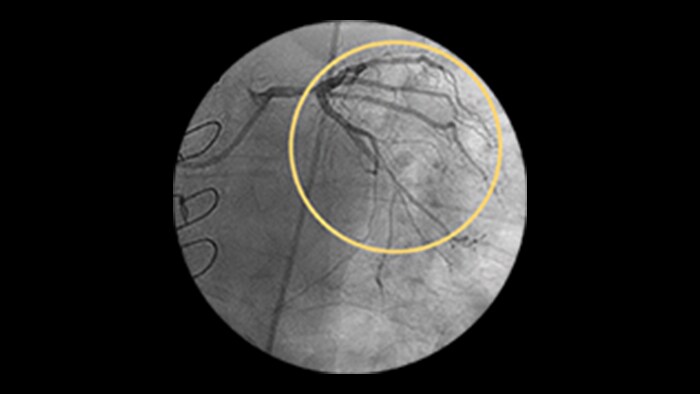
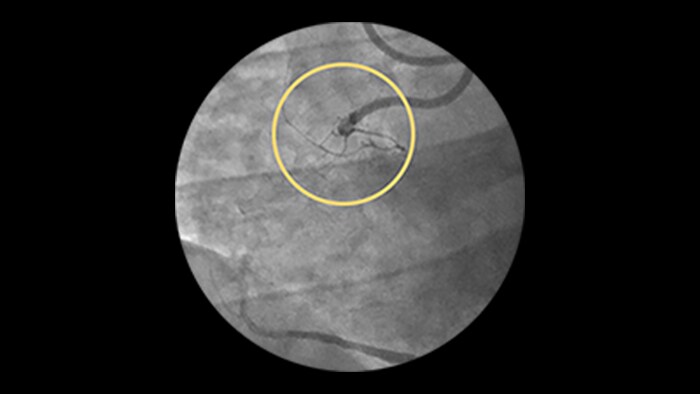
Dynamic Coronary Roadmap
This Philips-exclusive technology provides a real-time view of the coronary arteries and removes the need for additional contrast injections.
You are about to visit a Philips global content page
Continue -
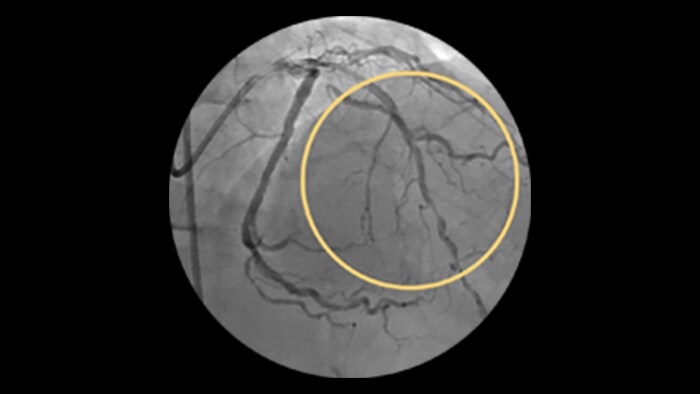
StentBoost
This simple, quick and cost-effective tool enhances stent visualization in the coronary arteries.
You are about to visit a Philips global content page
Continue
Explore clinical techniques for contrast reduction

Ultra-low contrast coronary angiography and zero-contrast percutaneous coronary intervention for prevention of contrast-induced nephropathy: step-by-step approach and review
Prof. Dariusz Dudek
President EAPCI ESC
Jagiellonian University
Krakow, Poland
Watch ultra-low contrast PCI contributions from around the world
Watch ULCPCI symposium at EuroPCR 2023
Watch ULCPCI by Dr. Abdul Mozid at ACI 2023
Watch ULCPCI symposium at EuroPCR 2022
Watch ULCPCI Symposium at EuroPCR 2021
Watch ULCPCI Symposium at EuroPCR 2020
Watch Prof. Escaned discuss Dynamic Coronary Roadmap for contrast reduction
Dr Al-Azizi explains why contrast reduction in PCI is important to him
Dr Kaki gives his tips on the best tools and techniques for ultra-low contrast PCI
New data shows that Philips Dynamic Coronary Roadmap has the potential to reduce the use of contrast agent during PCI procedures by 28.8%
Approach to contrast use in PCI
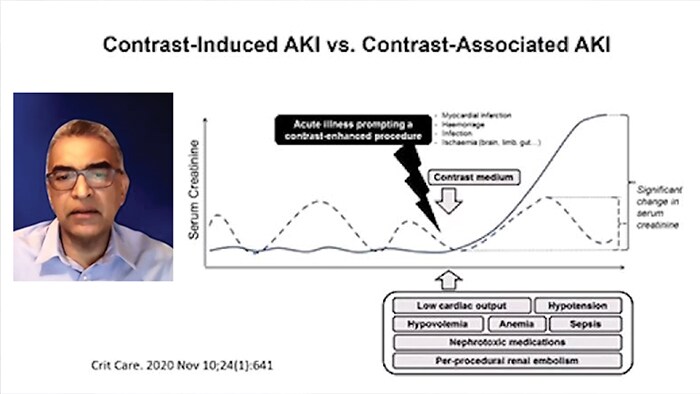
As low as reasonably achievable (ALARA) is a widely accepted approach to contrast use in PCI due to contrast induced acute kidney injury (CI-AKI).
Contrast medium has a "two-hit" model of direct contrast-induced renal tubular epithelial cell toxicity and vasoconstriction within the renal arteries that can last up to several hours, which results in the kidneys becoming ischemic and a decreased glomerular filtration rate (GFR), this in-turn causes a rise in creatinine. This complication adds to the patient’s length of stay and cost accrual to the hospital.
CI-AKI (also referred to as contrast induced nephropathy, CIN) is one of the major causes of hospital-acquired AKI:
New invasive imaging techniques and co-registration software allows PCI to be performed with limited amounts of contrast, even with zero-contrast use, to reduce the risk of CI-AKI for all patients, and especially those with renal insufficiency.
5.73% of patients leave the cath lab with AKI following PCI.5 Those with AKI had higher hospitalization cost than those without ($38,869, SD 42,583 vs $17,167 SD 13,994, p <0.001).
AKI was associated with an increase in hospitalization cost of $9,4485
AKI was associated with an increase length of stay of 3.6 days5
Every incremental 75ml of contrast used increased the risk of AKI by 42%15
1,349,612 patients who underwent PCI (CathPCI Registry)15
The American College of Cardiology’s National Cardiovascular Data Registry (NCDR) CathPCI registry has a low threshold (rise in serum creatinine of 0.3 mg/dL) for AKI and encourages quality improvements.
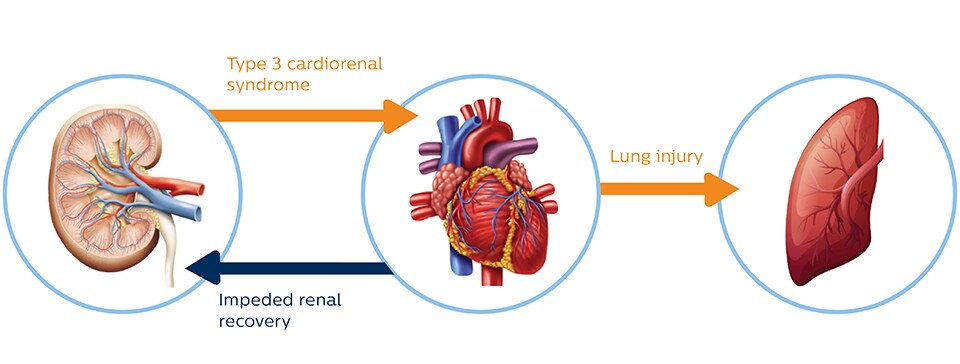
Cardiac and pulmonary consequence of AKI
AKI has been shown to promote cardiac injury and dysfunction, defined as type 3 cardiorenal syndrome. Heart failure, in turn, can impede renal recovery. Lung injury and hypoxemia after acute kidney injury can arise both from increased capillary permeability and from increased hydrostatic capillary pressure due to heart failure.6
High-risk CAD
Cardiovascular disease is the leading cause of death in patients with chronic kidney disease (CKD), stage IV-V, and end stage renal disease (ESRD) with the level of glomerular filtration rate (GFR) being an independent predictor of atherosclerotic cardiovascular disease.7
Cardiovascular risk odds ratio according to stage of CKD8*
| Stage | Estimated GFR | Cardiovascular risk |
| 1 | >90† | Dependent on degree of proteinuria |
| 2 | 30–89† | 1.5 |
| 3 | 30–59 | 2-4 |
| 4 | 15–29 | 4-10 |
| 5 | <15 | 10-50 |
| ESRD | Dialysis | 20-1000 |
∗ The increase in risk in comparison with people free from CKD depends on the age of the population studied: the younger the subject, the higher the relative risk. Microalbuminuria increases the cardiovascular risk by an additional 2- to 4-fold.
† Evidence of functional or structural kidney abnormalities for ≥3 months defined as abnormal renal biopsy, markers of renal damage (persistent proteinuria, albuminuria, hematuria) or structural renal abnormality on imaging studies.
CKD patients are widely excluded from RCT’s which leads to a paucity of data and makes cardiovascular management in this population very challenging.
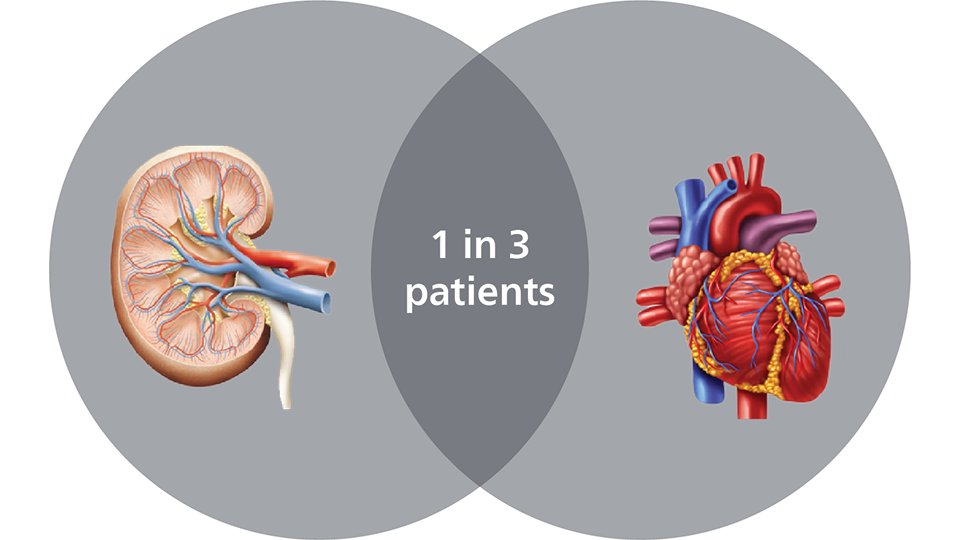
CKD patients with coronary artery disease pose a significant demographic to address, 30-60% (1 in 3)9 and have a significantly increased risk of CV mortality due to contrast induced nephropathy (CIN)
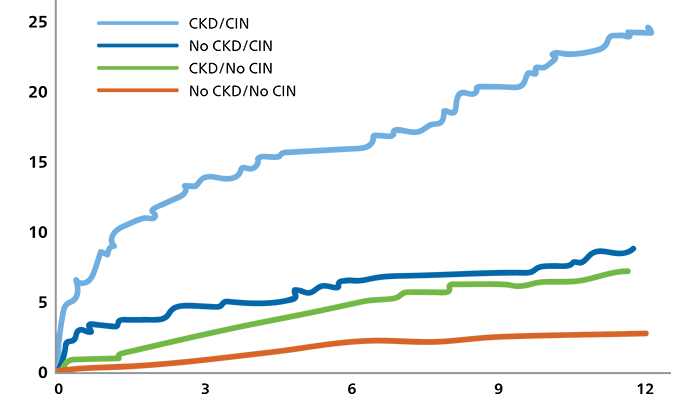
Mortality significantly increases at 1-year with CKD and CIN10
Renalism11: Many CKD patients will get deferred from PCI through fear of CIN
Despite a potential mortality benefit with coronary revascularization in patients with CKD presenting with ACS, diagnostic and therapeutic coronary interventions are under-utilized in patients with CKD, including those with ST elevation MI.
| Authors12 | Clinical presentation | Patients (N) | CKD | CKD invasive | No CKD invasive |
| Chertow et al | MI | 57,284 | 26% | 25% | 47% |
| Han et al | NSTE ACS | 45,343 | 14% | 48% | 74% |
| Goldenberg et al | NSTE ACS | 13,141 | 32% | 50% | 68% |
| Szummer et al | MI | 57,477 | 33% | 33% | 58%a |
| Nauta et al | MI | 12,087 | 25%b | NS | NS |
MI, myocardial infarction; NSTE, non-ST segment elevation; ACS, acute coronary syndrome. CKD defined based on eGFR<60 ml/min per 1.73 m²
a Denotes the percentage of patients with NSTEMI who underwent revascularization based on CKD status, defined as eGFR<60 ml/min per 1.73 m2.
b CKD defined based on eGFR<60 ml/min per 1.73 m2.
Preventing CI-AKI
A consensus exists for the beneficial effect of hydration in preventing CI-AKI. Hydration increases urine flow rates, reduces the concentration of contrast media in the tubule, and expedites excretion of contrast media, thus reducing the length of time that tubular cells are exposed to the toxic effects of contrast media.13 Patient hydration can be managed using the Poseidon trial protocol. Left ventricular end diastolic pressure (LVEDP) guided fluid administration. LVEDP is an important measure of ventricular performance and may identify patients at increased risk for developing late clinical symptoms of heart failure (HF).
3mL/kg bolus
LVDEP guided rate X 4hrs
| LVDEP | Rate |
| <13 | 5.0 ml/kg/hr |
| 13-18 | 3.0 ml/kg/hr |
| >18 | 1.5 ml/kg/hr |
Contrast volume
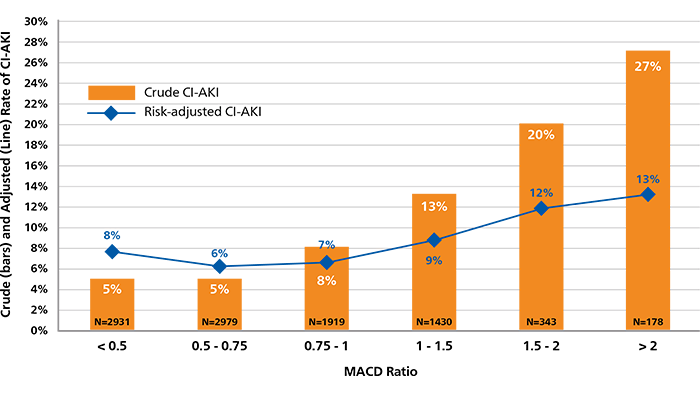
Contrast volume is a key risk factor for CI-AKI and matters the most in the highest-risk patient. The incremental use of contrast beyond the Maximal Allowable Contrast Dose (MACD) is associated with an increased risk of CI-AKI.14
MACD = Contrast volume/eGFR ratio ≤ 1.
MACD Ratio and CI-AKI. Calculated ratios of contrast volume to the predicted Maximal Allowable Contrast Dose (MACD) are plotted by the crude (orange bars) and risk-adjusted (black diamonds) rates of contrast-induced acute kidney injury (CI-AKI).
Explore more
Technology to support contrast reduction
Philips PCI guidance solutions incorporate non-invasive and adjunctive IVUS and iFR modalities, requiring less contrast for proper diagnosis.



References
1. Mehran R, Aymong ED, Nikolsky E, et al. A simple risk score for prediction of contrast induced nephropathy after percutaneous coronary intervention: development and initial validation. J Am Coll Cardiol. 2004; 44(7): 1393-1399. doi:10.1016/j.jacc.2004.06.068. 2. Wang, H. E., Muntner, P., Chertow, G. M., & Warnock, D. G. (2012). Acute kidney injury and mortality in hospitalized patients. American Journal of Nephrology, 35(4), 349–355. https://doi.org/10.1159/000337487. 3. Brown, J. R., Rezaee, M. E., Nichols, E. L., Marshall, E. J., Siew, E. D., & Matheny, M. E. (2016). Incidence and In-Hospital Mortality of Acute Kidney Injury (AKI) and Dialysis-Requiring AKI (AKI-D) After Cardiac Catheterization in the National Inpatient Sample. Journal of the American Heart Association, 5(3), e002739. https://doi.org/10.1161/JAHA.115.002739. 4. Mohammed, N. M., Mahfouz, A., Achkar, K., Rafie, I. M., & Hajar, R. (2013). Contrast-induced Nephropathy. Heart views: the official journal of the Gulf Heart Association, 14(3), 106–116. doi:10.4103/1995-705X.125926. 5. Amin P, et al. Incremental cost of Acute Kidney Injury after Percutaneous Coronary Intervention in the United States. The American Journal of Cardiology, 125(1), 29–33. https://doi.org/10.1016/j.amjcard.2019.09.042. 6. Legrand, M. & Rossignol, P. 2020. Review article: Cardiovascular Consequences of Acute Kidney Injury. N Engl J Med 2020;382:2238-47. DOI: 10.1056/NEJMra1916393. 7. Manjunath G, et al. Level of Kidney Function as a Risk Factor for Atherosclerotic Cardiovascular Outcomes in the CommunityJACC 2003; 41:47–55. 8. Edwards, N. et al. Defining the Natural History of Uremic Cardiomyopathy in Chronic Kidney Disease: The Role of Cardiovascular Magnetic Resonance. J Am Coll Cardiol Img. 2014 Jul, 7 (7) 703–714. 9. Coca SG, Krumholz HM, Garg AX, Parikh CR. Underrepresentation of renal disease in randomized controlled trials of cardiovascular disease. JAMA. 2006; 296(11):1377-1384. 10. Dangas G, et al. Contrast-Induced nephropathy after percutaneous coronary interventions in relation to chronic kidney disease and hemodynamic variables. AJC 2005. 11. Chertow et al. “Renalism”: Inappropriately Low Rates of Coronary Angiography in Elderly Individuals with Renal Insufficiency. J Am Soc Nephrol 15: 2462-2468, 2004. 12. Weisbord S. AKI and Medical Care after Coronary Angiography: Renalism Revisited. CJASN November 2014, 9 (11) 1823-1825; DOI: https://doi.org/10.2215/CJN.09430914. 13. Brar et al. Hemodynamic-guided fluid administration for the prevention of contrast-induced acute kidney injury: the POSEIDON randomized controlled trial. Lancet 2014. DOI: https://doi.org/10.1016/S0140-6736(14)60689-9. 14. Brown et al. Does Safe Dosing of Iodinated Contrast Prevent Contrast-Induced Acute Kidney Injury? Circ Interv 2010;3:346-350. 15. Amin, A. P., Bach, R. G., Caruso, M. L., Kennedy, K. F., and Spertus, J. A. (2017). Association of Variation in Contrast Volume With Acute Kidney Injury in Patients Undergoing Percutaneous Coronary Intervention. JAMA cardiology, 2(9), 1007–1012. https://doi.org/10.1001/jamacardio.2017.2156.