A commitment to shared knowledge and the advancement of medicine
Philips eICU programs generate an incredible amount of data on ICU patient stays every year. Philips eICU Research Institute (eRI), a non-profit institute established by Philips and governed by customers, is a platform built from a repository of data that is used to advance knowledge of critical and acute care.
For Philips customers that participate, eRI provides access to the most comprehensive database of ICU care in the world and opportunities for sponsored research and to participate in an initiative that has a significant impact on critical care. In addition, eRI directly benefits customer telehealth programs as research is frequently translated into new and advanced tools and programs.
Analytics which drive evidence-based best practice
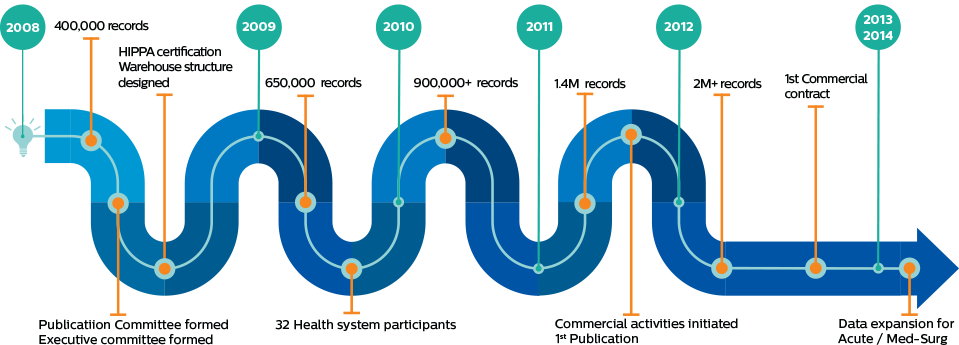
Philips eICU Research Institute (eRI) was established by Philips as a platform to advance the knowledge of critical care. The ERI database is a repository of anonymous data donated by member institutions and is instrumental in product development as well as a key enabler for critical research in the intensive care field.
Real world results
The impact of eRI extends from product development to critical research and beyond, enabling academia and member organizations to collaborate, innovate and advance critical care together. The following is just a sample of what’s been done and what’s possible with eRI: Product development – Improving Philips clinical decision support tools
Research – Improving critical care
Continually evolving, continually improving
Product development
Collaboration
Research



